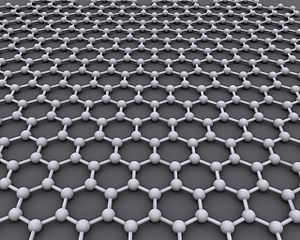
Carbon Sheet - Chemical Sensor
A new form of carbon called graphene was discovered by British scientists in 2004. It consists of a single layer of atoms bonded in a hexagon pattern, similar to chicken wire. The graphite component in pencil lead is a stack of many tiny layers of graphene. The two-dimensional carbon structure has unusual properties. For example, it is one of the strongest materials known. If a large sheet could be fabricated, it would take an elephant, balanced on a pencil, to break through the surface. Thus far, however, such sheets are not available. Graphene fragments have only been made on the microscopic level.
Graphene makes an excellent sensor for chemicals because it is entirely surface area, both top and bottom, with no interior volume. A single molecule adsorbed onto graphene changes its conduction of electricity in a measureable way. The conductivity of graphene can also be controlled with external electric fields. That is, the material can be turned on as a conductor, or turned off as an insulator. This behavior is the key to the semiconductors used throughout solid state electronics, and graphene eventually may replace silicon in modern technology.